Milestone #5 is reached successfully with the Sapien 3 TAVI Device FSI Model
SimInSitu Project has reached one of the major milestones which addresses the validation (VAL), uncertainty quantification (UQ) and sensitivity analysis (SA) of the Sapien 3 TAVI Device (S3) FSI Model for an in-vitro test setup. S3 FSI Model is developed by 4RealSim and employs the Smoothed Particle Hydrodynamics (SPH) method for modelling the fluid flow. One of the main advantages of the SPH method is that both structure and flow parts are handled by a single software, Abaqus, and coupling of different solvers is not needed.
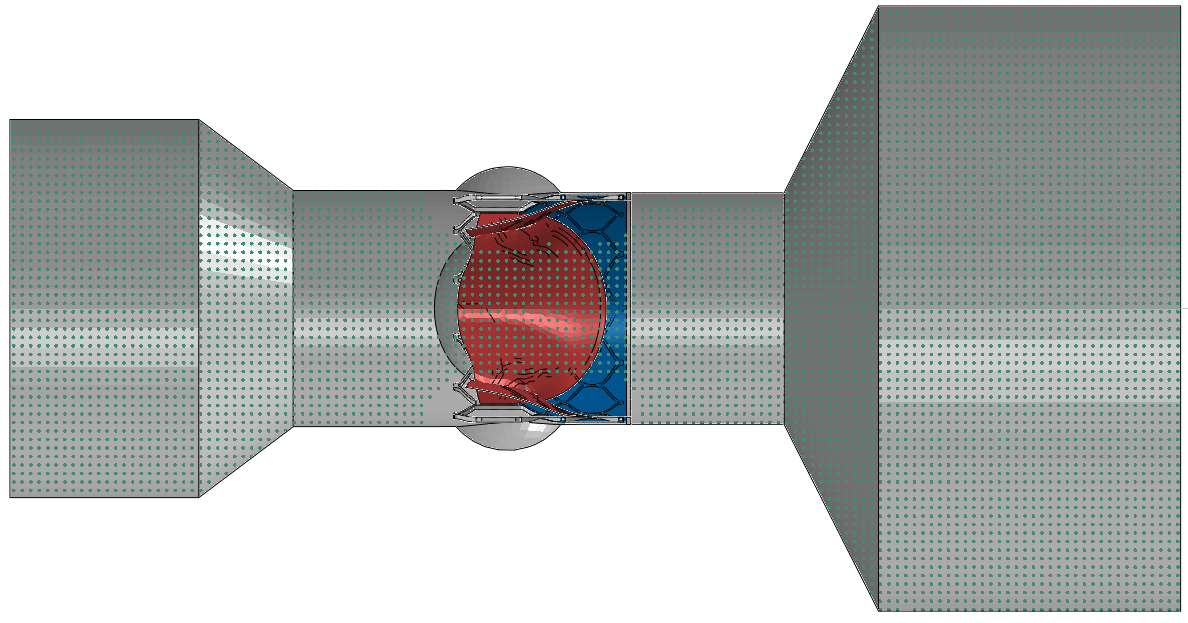
The in-vitro S3 SPH FSI Model is composed of a S3 device placed into an in-vitro test chamber which has two reservoirs connected to each end of the chamber, as shown below. During the systolic duration, the inflow reservoir acts as the source of SPH particles and the outflow reservoir acts as the collector. During the diastolic period, the roles are switched. With the user subroutines and boundary condition switching techniques developed specifically for this model, user has full control over the operational point (i.e., systolic and diastolic pressures at inlet and outlet, flow rate during systole, and heart rate).S3 SPH FSI model VAL is performed using 2 experimental reference cases that were available in the literature. The references uses the S3 devices with 26-mm [1] and 23-mm [2] nominal diameters having different expansion settings and place them in test chambers with sinuses. The chambers were connected to the hydro-pulser test setup where a specific cardiac cycle is applied. Same setups were created with the SPH FSI models and the same cardiac cycle conditions were simulated as discrete VAL comparisons as shown in the animations below.
In order to obtain model uncertainty, VAL assessment also included SA&UQ according to ASME V&V 20 standard, where the uncertainty is calculated with a linear Taylor series expansion in parameter space assuming the input parameters are uncorrelated. VAL assessment is done for several output parameters using the area metric from the cumulative distribution function (CDF) curves which enables to account for the model uncertainty as well. VAL assessment showed that the SPH FSI model is predicting the experiments in a good agreement with an error of less than 10% for most of the output parameters.In conclusion, targeted VAL requirement of maximum 10% error is achieved for most of the results. Considering the unknowns of the experiments, it is shown that the S3 in-vitro SPH FSI model is predicting the flow response in a good agreement with the experiments. It is shown that the developed SPH model is robust and can be used to simulate the device behavior in the subsequent work-package (WP4) in a patient-specific environment.
References:
1.Madukauwa-David ID, Sadri V, Kamioka N, Midha PA, Raghav V, Oshinski JN, Sharma R, Babaliaros V, Yoganathan AP. Transcatheter aortic valve deployment influences neo-sinus thrombosis risk: An in vitro flow study. Catheter Cardiovasc Interv. 2020 Apr 1;95(5):1009-1016. doi: 10.1002/ccd.28388. Epub 2019 Jul 9. PMID: 31287238.
2. Brennan J. Vogl, Ahmed El Shaer, Juan A. Crestanello, Mohamad Alkhouli, Hoda Hatoum. Flow dynamics in the sinus and downstream of third and fourth generation balloon expandable transcatheter aortic valves. Journal of the Mechanical Behavior of Biomedical Materials, Volume 127, 2022, 105092, ISSN 1751-6161, https://doi.org/10.1016/j.jmbbm.2022.105092.